Molecular Orbitals, created jointly by postdoctoral researcher Pierpaolo Morgante
and professor Jochen Autschbach, and published by American Chemical Society
(ACS) Publications, Washington, DC (2023), is a primer on the topic of Molecular
Orbitals (MOs): What are MOs, how can you calculate them, how can you
visualize MOs and the associated atomic orbitals (AOs), how are MO concepts
used in chemistry, and how do you avoid common misconceptions about
MOs?
Molecular Orbitals is part of the ACS In Focus digital primers series (see also the
In-Focus Google Play page). The In-Focus series is aimed at readers of all levels who
want to acquire a fundamental understanding of core topics and techniques in
chemistry and adjacent fields. In this spirit, we created a primer about MO theory
and its applications that will go a long way to help you understand this important
concept in chemistry.
This primer can be read in about 7–8 hours. It treats the topic rigorously, but derivations are skipped in favor of outlining how you get from one level of approximation to the next. Detailed derivations of MO-theoretical concepts and many additional aspects of electronic structure theory are covered in Jochen’s textbook Quantum Theory for Chemical Applications.
Molecular Orbitals comes with 50+ high-quality figures illustrating the concepts that are presented. For many of the illustrations, we provide interactive versions on this page for download. Details are provided below.
Please scroll down for a selection of free supplementary material for the
eBook.
Below is a list of notebooks in the Wolfram computational language (aka
Mathematica syntax) for download. We used Mathematica to create most of the
function plots and molecular graphics shown in the ebook. The list of notebooks is
ordered by chapter, with self-explanatory file names.
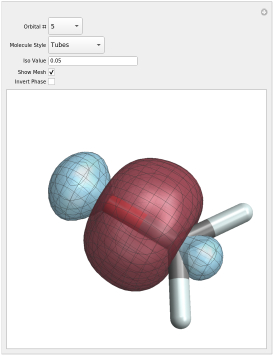
The notebooks (file extension .nb) can be viewed with Mathematica, and with the
free Wolfram Player. Mathematica is not free software, but many colleges have
campus licenses, which means that students and instructors can use it without
having to pay for an individual license. The Wolfram Player is free for download, runs
on Windows, Mac, and Linux operating systems, and it will faithfully reproduce the
visualizations and almost all of their interactive features. The main drawback of
using the Player is that you won’t be able to modify the underlying code
easily.
Most of the files contain embedded volume data for several orbitals. Therefore, the
file size is typically several 10 MByte. Also, please be patient when the isosurface
plots get rendered in the software. When you open one of the files, please enable
dynamic content if prompted.
Note: Visualization functionality was confirmed to work with Mathematica and
Wolfram Player versions 13.0 and 13.3, and very likely the software works also with
any of the release 12 and 14+ versions. Mathematica and Wolfram Player version
13.1 have a bug and will not produce correct images. Versions 13.2 might
produce images that appear as if they were based on low-resolution data.
Chapter 4: Function Plots in 1d, 2d and 3d (11 MB)
Chapter 4: H2CO Canonical Orbitals (47 MB)
Chapter 5: H2CO Localized Orbitals Boys Criterion (49 MB)
Chapter 5: H2CO Localized Orbitals PM Criterion (47 MB)
Chapter 5: Benzene Canonical Orbitals (51 MB)
Chapter 5: Benzene Localized Orbitals PM Criterion (68 MB)
Chapter 5: Hexatriene Canonical Orbitals (79 MB)
Chapter 5: Hexatriene Localized Orbitals PM Criterion (75 MB)
Chapter 7: Ethene Canonical Orbitals (13 MB)
Chapter 7: Cyclobutane Canonical Orbitals (36 MB)
Chapter 7: Butadiene Canonical Orbitals (41 MB)
Chapter 7: Cyclohexene Canonical Orbitals (65 MB)
Chapter 7: Hexaaminecobalt(III) Canonical Orbitals (112 MB)
Chapter 7: Cobalt Pincer Complex Canonical Orbitals (81 MB)
Chapter 7: Ferrocene Canonical Orbitals (75 MB)
Chapter 7: Pt-Helicene (neutral form) HOMO and LUMO (117 MB)
Chapter 7: Pt-Helicene (protonated form) HOMO and LUMO (148 MB)
© 2025 J. Autschbach.